Introduction
Neuromodulation is a mechanism in the nervous system which alters the way nerve cells communicate and behave. This process is crucial for adapting organism behavior to different environmental stimuli, enhancing survival. In neurobiology, understanding neuromodulation not only helps identify the basic principles of brain function but also assists in developing therapeutic treatments for neurological disorders.
Aplysia californica, a large sea slug, has been a model organism in the study of neuromodulation, primarily due to its relatively simple nervous system, which consists of large, easily identifiable neurons. This simplicity allows for detailed studies of cellular mechanisms that impact learning and memory. Eric Kandel’s research on Aplysia has demonstrated how changes in synaptic strength result in learning and memory, highlighting the role of neuromodulation in these processes. His work has been important in advancing our understanding of how memory functions at a molecular level, particularly through his studies with Aplysia (Robertson & Walter, 2010).
Studying neuromodulation in Aplysia involves various techniques that range from molecular biology to behavioral neuroscience. By examining how specific neuromodulators, like serotonin, affect synaptic plasticity, researchers have gained insights into the general principles by which neurons alter their responsiveness to synaptic inputs. These findings have broad implications, informing our understanding of more complex brains, including humans (Orvis et al., 2022).
Exploring neuromodulation in Aplysia offers valuable lessons on the impact of environmental changes on neural circuits and behavior. For instance, how does Aplysia adapt its behavior in response to changes in the availability of food or the presence of predators? These adaptive mechanisms, driven by neuromodulatory processes, are necessary for the survival of the organism and are reflective of similar adaptations in higher organisms (Yang et al., 2016).
Aplysia not only enriches our understanding of basic neuroscientific concepts but also provides insights into the potential for applying this knowledge to treat human diseases. As this field advances, it holds the promise of revealing more about the intricate dance between neural function, behavior, and environmental factors.
What is Neuromodulation?
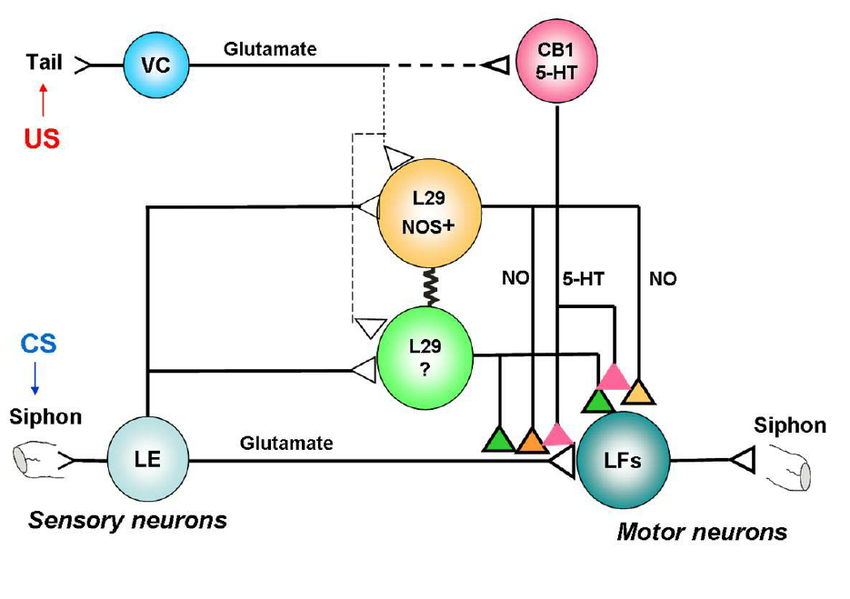
Neuromodulation is the process where a neuron uses one or more chemicals to regulate diverse populations of neurons, altering the way these cells communicate and respond to stimuli. Unlike classical synaptic transmission, where the communication between neurons is direct and relatively fast, neuromodulation involves neurotransmitters that diffuse through neural tissue, affecting a broad area and modulating the activity of various neurons within that region. This type of signaling is less about direct transmission and more about adjusting the overall neural environment, which can influence numerous neurons simultaneously, leading to a more coordinated and gradual adjustment in neural activity (Nadim & Bucher, 2014).
Neuromodulation encompasses a wide range of biochemical mechanisms that serve to fine-tune the nervous system’s response to stimuli, making it incredibly versatile and necessary for maintaining neural health and function. Neuromodulators typically act on a variety of receptor types scattered across different neurons, each capable of initiating complex intracellular processes that can profoundly change the physiological properties of these cells. These changes can include altering the likelihood of a neuron firing in response to a given input, changing gene expression to adapt to long-term demands, or adjusting the strength and efficacy of synaptic connections, which are essential for neural plasticity and long-term potentiation (Nadim & Bucher, 2014).
Neuromodulators are often released from neural projections that can span large areas of the brain, affecting many neurons at once. This widespread effect is crucial for coordinating the activity of different parts of the brain, enabling complex behaviors and responses to environmental stimuli. For example, dopamine, a well-known neuromodulator, plays a significant role in areas of the brain involved in movement, motivation, and the reward system. Its modulation of neural activity is essential for both normal function and in the context of various neurological disorders, where dopamine levels may be aberrant (Klein et al., 2019).
One of the key features of neuromodulation is its ability to impact the overall tone or state of brain regions, which can have profound effects on an individual’s mood, arousal state, and general neurological health. For instance, serotonin, another major neuromodulator, is heavily involved in mood regulation and overall brain excitability. Fluctuations in serotonin levels can lead to significant changes in mood and behavior, which is why this neuromodulator is a primary target for many antidepressant drugs. The ability of neuromodulators like serotonin to broadly adjust brain state makes them useful in both normal and pathological brain function (Peters et al., 2021).
Neuromodulation also plays a role in the brain’s ability to adapt to new information or environments, a process known as neural plasticity. This adaptability is required for learning and memory, where neuromodulators like acetylcholine and dopamine modify the strength of synaptic connections to enhance the storage and retrieval of information. This is particularly evident in studies of the hippocampus, a brain region involved in forming new memories, where neuromodulators facilitate the encoding of new experiences and the flexible retrieval of existing ones, allowing for dynamic and adaptable memory functions (Picciotto et al., 2012).
In the context of Aplysia californica, a model organism with a relatively simple nervous system, neuromodulation provides a unique window into the cellular mechanisms of learning and memory. Researchers can observe changes in synaptic function caused by neuromodulators in real-time, offering insights that are difficult to obtain in more complex organisms. The simplicity of the Aplysia nervous system allows for detailed mapping of neuromodulatory effects, providing a clearer understanding of how these processes can influence cellular and behavioral responses to environmental stimuli (Kandel, 2001b).
Neuromodulation in Aplysia has revealed that even simple neural circuits are capable of sophisticated behaviors due to the dynamic interplay between neuromodulators and neural activity. For example, the release of serotonin in Aplysia can lead to an enhancement of sensory neuron responses, facilitating a form of learning known as sensitization, where the organism becomes more responsive to certain stimuli based on previous experiences. This is a key example of how neuromodulation can alter behavior in significant ways, even in simple organisms (Barbas et al., 2003).
Neuromodulation is not just limited to the central nervous system but also occurs extensively in the peripheral nervous system, where it regulates a wide array of bodily functions such as muscle contraction, heart rate, and digestion. These peripheral neuromodulatory processes play a critical role in maintaining homeostasis and enabling the body to adapt to internal and external changes. The ubiquitous nature of neuromodulatory processes highlights their importance across different biological systems and highlights their evolutionary significance (Breit et al., 2018).
The implications of neuromodulation are vast and extend beyond neuroscience into fields such as psychology, pharmacology, and even artificial intelligence. Understanding how neuromodulators work can help inform treatments for a variety of conditions, from mental health disorders to neurodegenerative diseases, and can inspire the development of bio-inspired computational models for artificial intelligence systems. These models could mimic neuromodulatory processes to create more adaptive and robust AI systems, potentially transforming technology and medicine (Teufel & Fletcher, 2016).
Neuromodulation is an aspect of how the nervous system operates, integrating multiple signals to produce coherent and adaptive responses. Its study not only helps us understand the brain’s complexity but also opens pathways to therapeutic interventions that could ameliorate or cure many diseases. The broad impact of neuromodulation across different fields of study illustrates its importance as a central theme in understanding biological regulation and adaptability.
Neuromodulation in Biological Systems
Neuromodulation is a phenomenon in biological systems, extending its influence beyond the brain to virtually every part of the body. It plays a critical role in regulating a wide range of physiological processes, from the basic cellular level to complex behavioral responses. In the nervous system, neuromodulation adjusts the properties of neural circuits, thereby influencing how signals are processed and how organisms respond to their environment. This modulation is essential for the adaptability of organisms, allowing them to adjust their behavior in response to changing conditions and to maintain homeostasis (Moses et al., 2023).
Neuromodulation has been shown to affect the activity of immune cells, influencing inflammation and immune responses. Neurotransmitters such as acetylcholine and norepinephrine can modulate the activity of immune cells through specific receptors, which can alter the course of immune responses and affect the responses of diseases characterized by immune dysfunction. This cross-talk between the nervous system and the immune system highlights the extensive reach of neuromodulatory mechanisms, suggesting that they play a central role in coordinating the body’s response to both internal and external challenges (Sundman & Olofsson, 2014).
The endocrine system is another key area where neuromodulation exerts significant control. Hormones themselves can act as neuromodulators, influencing brain function and behavior, while the brain, in turn, regulates hormonal outputs through neuromodulatory signals. This bidirectional communication ensures that the body’s hormonal environment is finely tuned to meet the demands of various physiological states and environmental interactions. For instance, stress triggers the release of corticotropin-releasing hormone from the hypothalamus, which then stimulates the secretion of cortisol, a hormone that prepares the body to respond to stress (Leng, 2018).
Regarding behavior, neuromodulation is fundamental in shaping both innate and learned behaviors. By modulating the strength and output of neural circuits, neuromodulators like dopamine and serotonin can influence a wide range of behaviors from mood and motivation to social interaction and decision-making. These substances allow the nervous system to adapt behavioral outputs in a context-dependent manner, providing the flexibility needed to navigate complex social environments and to learn from past experiences (Rogers, 2011).
Neuromodulation also influences the development of the nervous system. During development, neuromodulators can influence the growth and differentiation of neurons, the formation of neural circuits, and the plasticity of synapses. These effects are crucial for the proper formation of the nervous system and can have lasting impacts on an individual’s ability to process information and respond to the environment throughout their life (Maloney, 2021).
Neuromodulation in biological systems also has significance for medicine and therapy. By understanding how neuromodulators influence various biological systems, researchers can develop targeted therapies that modulate these systems in beneficial ways. For example, neuromodulatory drugs are central to the treatment of neurological and psychiatric disorders, including depression, schizophrenia, and Parkinson’s disease. These treatments often aim to restore the balance of neurotransmitters in the brain, thereby alleviating symptoms and improving quality of life (Avery & Krichmar, 2017).
Neuromodulation is a biological process that integrates multiple systems within the body, facilitating a coordinated response to a myriad of physiological and environmental challenges. The study of neuromodulation not only deepens our understanding of how biological systems are interlinked and regulated but also opens up new avenues for therapeutic interventions. As research in this field progresses, it promises to reveal even more about the complex interplay between different systems, potentially leading to breakthroughs in our approach to health and disease.
Eric Kandel’s Research

Eric Kandel’s research has been important in advancing our understanding of how memory works at a molecular level, particularly through his studies with the sea slug Aplysia californica. Kandel’s work demonstrated that short-term and long-term memories are formed by different mechanisms within the nerve cells. His experiments revealed that short-term memory involves transient changes in the strength of preexisting connections between nerve cells, known as synapses, while long-term memory requires the synthesis of new proteins and the growth of new synapses (Riegel, 2020).
One of Kandel’s significant contributions was elucidating the role of synaptic plasticity in learning and memory. He discovered that learning leads to changes in the structure and function of synapses, which are the points of communication between neurons. This process, known as synaptic plasticity, is a mechanism underlying learning and memory. Kandel’s experiments showed that repeated stimulation of certain neural pathways could strengthen these pathways, a phenomenon known as long-term potentiation (LTP). This strengthening effect was shown to be dependent on the increased activity of certain enzymes and the expression of specific genes (Bailey et al., 2015).
Kandel also explored the biochemical pathways that underlie the storage of memory. His research identified a critical protein called CREB (cAMP response element-binding protein) that plays a key role in the formation of long-term memories. Activation of CREB leads to changes in gene expression that strengthen synaptic connections. This discovery provided a molecular link between transient signals in the nervous system and long-lasting changes in neural behavior, bridging the gap between short-term and long-term memory formation (Bickle, 2021).
In addition to his work on memory, Kandel’s research assists in our understanding of the biological basis of psychiatric disorders. His findings have suggested that some forms of mental illness, such as depression and anxiety, can be attributed to the malfunctioning of synaptic connections in the brain. This has opened up new avenues for therapeutic interventions, including the development of drugs that can specifically target synaptic pathways to restore normal brain function (Kandel, 2001a).
Kandel’s contributions to neuroscience have been widely recognized and celebrated, culminating in his receipt of the Nobel Prize in Physiology or Medicine in 2000. His work not only deepened our understanding of the physiological basis of memory but also laid the groundwork for exploring how alterations in brain function can lead to neurological and psychiatric disorders. His ongoing research continues to influence a wide range of fields, from cognitive psychology to neurobiology, and remains central to the ongoing quest to decipher the complexities of the human brain.
The Nobel Prize and Kandel’s Contribution
Eric Kandel’s groundbreaking work on the physiological basis of memory storage in the nervous system earned him the Nobel Prize in Physiology or Medicine in 2000. This prestigious award was in recognition of his innovative research on Aplysia californica, which elucidated how changes at the synaptic level contribute to learning and memory. Kandel’s findings demonstrated that learning produces changes in the strength of synapses—the junctions across which nerve cells communicate. His work provided compelling evidence for the synaptic plasticity theory of learning and memory, altering our understanding of how memories are formed, stored, and recalled.
The Nobel Prize highlighted not only the significance of Kandel’s discoveries but also highlighted the importance of using simple model organisms to unravel complex biological processes. By focusing on Aplysia, a sea slug with a relatively simple nervous system, Kandel was able to trace the specific changes that occur in the neural circuitry during learning. His approach demonstrated that even simple organisms can exhibit sophisticated behaviors and that the mechanisms underlying these behaviors can offer insights into the workings of more complex brains, including those of humans.
Kandel’s research journey began with an interest in the cellular mechanisms of learning and memory, which led him to explore the role of synaptic plasticity. He discovered that repeated stimulation of the sensory neurons in Aplysia led to increased neurotransmitter release and strengthening of synaptic connections, a phenomenon now known as long-term potentiation (LTP). This process was shown to be important for the formation of long-term memories and highlighted the plastic nature of the synapse.
Exploring the molecular mechanisms underlying synaptic plasticity, Kandel identified a protein kinase enzyme that plays a critical role in modulating synaptic strength. His team showed that activation of this enzyme leads to the phosphorylation of other proteins and changes in gene expression that strengthen synaptic connections. This cascade of biochemical events provided a molecular basis for understanding how short-term memories are converted into long-term memories, bridging the gap between transient experiences and lasting neural changes (Yasuda et al., 2022).
Kandel’s Nobel Prize-winning work also delved into the role of specific genes and proteins in memory formation. He identified the CREB (cAMP response element-binding protein) as a key player in the synthesis of new proteins necessary for long-term memory storage. This discovery was pivotal, as it linked molecular processes directly to the physiological changes observed in neurons during learning and memory formation.
Kandel’s findings extend beyond neuroscience, influencing fields such as psychology, psychiatry, and education. By elucidating the mechanisms of memory formation, his work has contributed to better understanding of cognitive disorders and has informed approaches to learning and teaching. It has also paved the way for the development of pharmacological treatments aimed at enhancing or mitigating memory formation in conditions like Alzheimer’s disease, PTSD, and learning disabilities (Pittenger, 2013).
Kandel’s contributions to neuroscience have fostered a deeper understanding of the mind-brain relationship, challenging and expanding the boundaries of how we define learning and memory. His interdisciplinary approach, which integrates molecular biology, psychology, and cognitive science, has helped build bridges between these fields, fostering collaborations that have propelled forward our understanding of mental processes.
Reflecting on his Nobel Prize, Kandel has emphasized the broader philosophical and ethics of his work. He has engaged in public discussions about the impact of neuroscience on our understanding of free will, the nature of self, and the potential for manipulating memories. These conversations highlight the societal relevance of his research and its potential to influence not only science but also aspects of human life.
Since receiving the Nobel Prize, Kandel has continued to be a leading figure in neuroscience. He has written extensively, educating the public about the brain and its functions through books and lectures. His ongoing research continues to influence new generations of neuroscientists, ensuring that his legacy will endure in the scientific community.
Implications and Future Directions
The research on neuromodulation, particularly through the lens of Eric Kandel’s work with Aplysia californica, has opened up numerous avenues for future exploration and application, especially in the realm of neural diseases. As we continue to unravel the complex mechanisms of synaptic plasticity and memory formation, there is a promising potential to apply these insights to understand and treat a variety of neural disorders. Diseases such as Alzheimer’s, Parkinson’s, and other forms of dementia could potentially be managed or even mitigated through targeted neuromodulatory therapies that restore synaptic function and enhance neural plasticity. The ongoing research into the molecular pathways involved in memory storage and retrieval provides a critical foundation for developing drugs that can precisely influence these pathways, offering hope for patients and families affected by these debilitating conditions (Hawkins, 2013).
Another significant area of future research is the application of neuromodulatory principles to the development of artificial intelligence and machine learning systems. By mimicking the adaptive and plastic nature of biological neural networks, researchers are exploring the creation of more flexible and efficient AI systems. These systems could potentially learn and adapt in ways that are inspired by the human brain, leading to advancements in how machines perceive, interact with, and respond to their environment. The intersection of neuroscience and computer science, fueled by our understanding of neuromodulation, promises to not only advance technological capabilities but also provide deeper insights into the operations of the human mind (Vecoven et al., 2020).
The ethical implications of neuromodulation research are also profound and warrant careful consideration as we move forward. As we gain more control over the brain’s functioning through neuromodulatory techniques, questions about the manipulation of memory, cognition, and identity become increasingly relevant. These issues touch on the core of what it means to be human, challenging our concepts of autonomy and the ethical limits of scientific intervention. It is important that as we advance in our capabilities, we also develop robust ethical frameworks to guide the application of these powerful technologies.
In addition to therapeutic applications, the principles of neuromodulation hold significant promise for educational strategies. Understanding how neuromodulators affect learning and memory can lead to more effective educational techniques that are tailored to the physiological needs of learners. This could revolutionize traditional educational paradigms, making learning more personalized and effective, thereby enhancing educational outcomes across diverse populations. The potential to apply neuromodulatory insights in educational settings opens up exciting possibilities for fostering lifelong learning and cognitive resilience.
The role of neuromodulation in aging and longevity is another critical area of research that could profoundly impact our understanding of health and aging. As the global population ages, there is an increasing need to understand how neuromodulatory processes change over time and how these changes affect cognitive function and general well-being. Research in this area could lead to interventions that enhance brain health in the elderly, potentially extending cognitive vitality and reducing the incidence of age-related neurological disorders.
The exploration of neuromodulation in Aplysia and other model organisms continues to provide insights into the universal principles of neural function. These studies not only deepen our understanding of the brain but also contribute to our knowledge of evolutionary biology, showing how complex behaviors and sophisticated neural mechanisms have evolved across different species. This comparative approach helps to highlight the shared biological heritage among diverse organisms and demonstrates the interconnectedness of life on Earth.
Conclusions
The exploration of neuromodulation in Aplysia californica has significantly advanced our understanding of neural mechanisms underlying learning and memory. Eric Kandel’s work, which earned him the Nobel Prize, has not only deepened our comprehension of synaptic plasticity but also set a foundation for exploring therapeutic approaches to neural disorders. This research is vast, extending beyond the confines of neurobiology to touch on areas such as mental health, cognitive enhancement, and the treatment of neurodegenerative diseases. As we continue to uncover the intricate details of neuromodulatory processes, we are better equipped to develop interventions that can ameliorate conditions like Alzheimer’s and Parkinson’s disease, potentially transforming the lives of millions.
The principles derived from studying neuromodulation in simple organisms like Aplysia are informing the development of more sophisticated technologies, including artificial intelligence. By mimicking the adaptive capabilities of biological neural networks, we can enhance the flexibility and efficiency of AI systems. This cross-disciplinary influence not only propels technological innovation but also provides a deeper understanding of human cognition, offering insights that are important for both advancing AI and improving human-computer interactions.
The ethical considerations arising from neuromodulation research also demand our attention. As we gain the ability to manipulate neural functions more precisely, we must carefully consider the implications of such power. The potential to alter memory, mood, and behavior brings with it significant ethical challenges that must be addressed to ensure that neuromodulatory technologies are used responsibly. Developing comprehensive ethical guidelines will be essential as we navigate the complex landscape of neuroscience and its applications in medicine, technology, and beyond.
In conclusion, the study of neuromodulation holds transformative potential for numerous fields. From providing new strategies for treating mental and neurological disorders to influencing the next generation of technological advancements, the insights gained from this research are shaping the future of science and society. As we move forward, it is likely that we continue to explore these mechanisms with a thoughtful consideration of both their scientific possibilities and their broader societal impacts.